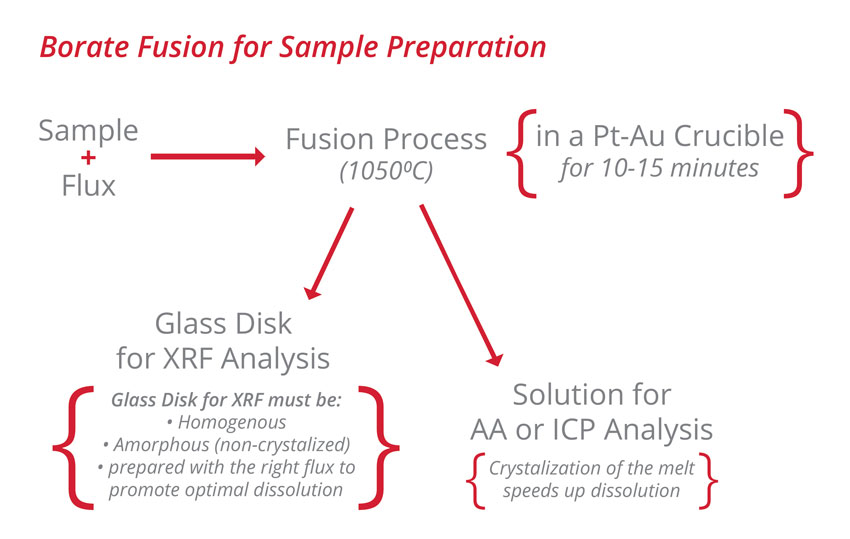
Borate fusion is a widespread sample preparation method for XRF, AA and ICP analysis. It outperforms many other procedures for the preparation of a wide range of materials including: carbonates, catalyst, cement, ceramic, ferroalloys, geological samples, glass, pure metals, silica and slag.
For XRF analysis, the production of an homogenous glass disk reduces effects associated with particle size, mineralogy and matrix to obtain better accuracy than pressed powders.
For solutions, borate fusion is a rapid and safe method to eliminate the use of concentrated and dangerous acids (such as HF) in sample preparation. The preparation time is shorter than conventional acid digestions. When using high purity fusion fluxes and appropriate analytical parameters, the fusion can easily become a powerful routine technique to prepare a wide range of samples.
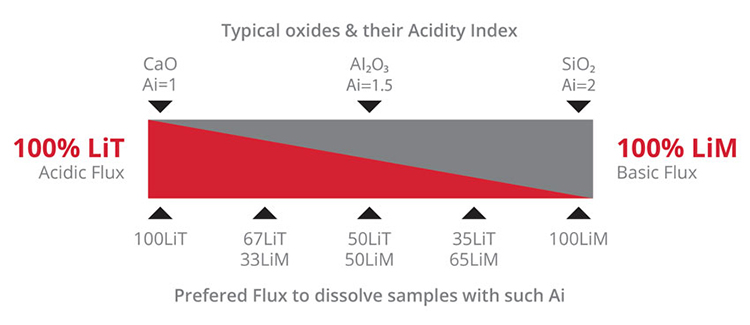
Fusion is a method where an oxidized sample is dissolved in the molten flux at temperatures of around 1050⁰C. For optimal dissolution, the right flux composition must be used. Two basic formulations, lithium tetraborate (LiT or Li₂B₄O₇) and lithium metaborate (LiM or LiBO₂) are commonly used in various proportions.